Better safe than sorry!
Component manufacturing and devices for medical technology
 For many decades, SEDLBAUER AG has been an indispensable partner of renowned manufacturers in the medical technology sector. Our expertise in sheet metal fabrication and electrical engineering allows us to supply our customers with high quality medical components and equipment. Our quality management system according to ISO 13485 guarantees you the highest quality and product safety.
For many decades, SEDLBAUER AG has been an indispensable partner of renowned manufacturers in the medical technology sector. Our expertise in sheet metal fabrication and electrical engineering allows us to supply our customers with high quality medical components and equipment. Our quality management system according to ISO 13485 guarantees you the highest quality and product safety.
High quality standards according to EN ISO 13485, extensive controls and continuous process optimization
Equipment in hospitals, rehabilitation centers or medical practices must meet the highest quality and safety standards. Compliance with applicable standards, innovative strength, but also trust and experience play an important role. We manufacture medical devices and components with a quality you can rely on. Extensive controls during production and continuous optimization of our processes are a matter of course for us. The entire development and manufacturing process is documented by us. This includes complete traceability from the starting material to the finished product as well as plant qualification and process validation.
Own products and customized solutions
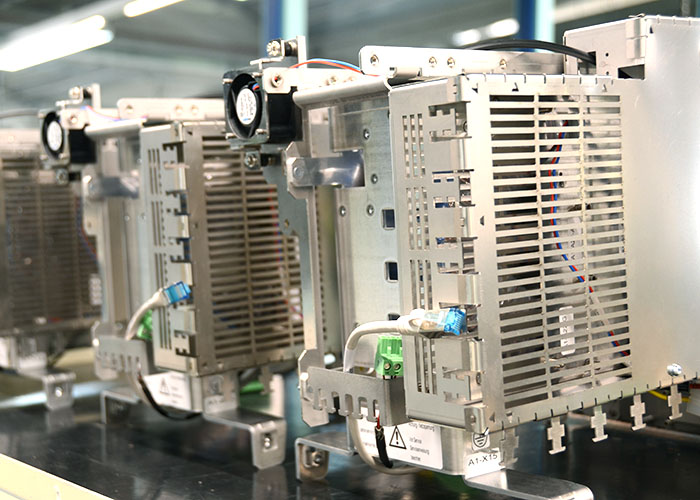
In addition to our own products (medical isolating transformers & accessories), we also create individual partial and complete solutions for our customers. Our portfolio ranges from project planning, development and design to production and service.
To the point:
|
|
|
|
|
|
|
|
|
|
|
Thanks to our many years of experience in sheet metal processing and electrical engineering and our EN ISO 13485 certification, we supply our customers with high-quality medical components and devices that they can trust.
Because the safety of the operating personnel and the patients does not deserve any compromises.

Christina Friedl
Product Management Electromechanical Systems, Medical Engineering
+49 (0)8552 41-111
c.friedl@sedlbauer.de

Julian Müllner
Product Management Power Units, Medical Engineering, E-Mobility
+49 (0)8552 41-131
j.muellner@sedlbauer.de
Certified according to:
- EN ISO 13485
- EN 60601
- NRTL Certificate
- REACH Declaration of Conformity
- RoHS Declaration of Conformity
- All certificates
Medical devices – quality management systems (certified since 01.04.1995)
The EN 60601 series of standards defines safety requirements and ergonomic demands for medical electrical equipment and in medical systems. It is based on the internationally valid IEC 60601.
for MTT 1000 and polyMIT
Product examples
Own products
Medical isolating transformers

polyMIT series with NTC inrush current limitation

polyMIT series "e%with electronic inrush current limitation

MTT 1000 certified according to UL 2601-1
What is the difference between the polyMIT and polyMITe series?
Whereas in the original polyMIT family the starting current of the toroidal transformer is limited mechanically with an NTC, the polyMITe series has an electronic inrush current limitation including half-wave failure detection.
What is the difference between the polyMIT series and the MTT?
Due to its three taps, the MTT1000 can be operated with 100V in addition to 115 and 230V (note: the permissible power output drops to approx. 850VA). Furthermore, the MTT1000 has additional overload protection on the secondary side.
Customers:
- ME equipment manufacturer
- ME systems planner
- Medical technology specialist dealers
- Series and special solutions for customer-specific applications
User:
- Clinics
- Hospitals
- Medical practices
- Diagnostic centers
- Therapy and rehabilitation centers, etc.
Customized solutions

Housing for laboratory technology
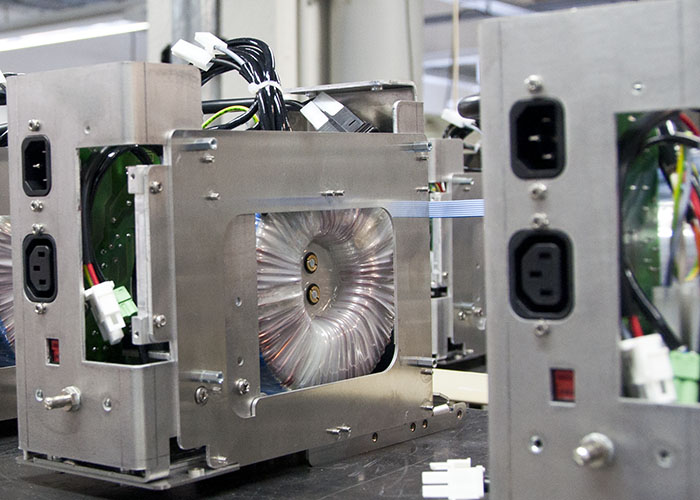
Electromechanical assembly for medical technology

Chassis for eye surgery
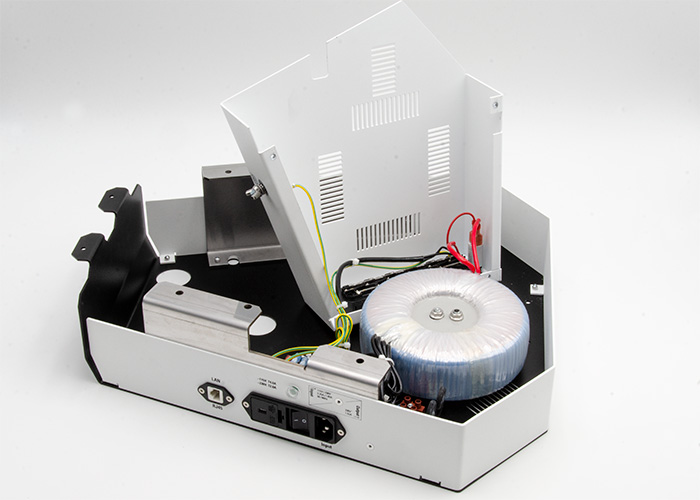
Power supply for skin cancer diagnostic device